Structure-guided Antibody Cocktail for Prevention and Treatment of COVID-19
- 作者:Shih-Chieh Su, Tzu-Jing Yang, Pei-Yu Yu, Kang-Hao Liang, Wan-Yu Chen, Chun-Wei Yang, Hsiu-Ting Lin,
- 期刊: 本篇研究論文於2021年10月22日正式發表在國際期刊《PLOS病原體》 https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009704
抗體雞尾酒療法! 對抗新冠肺炎變異病毒株潛力
新型冠狀病毒 (SARS-CoV2)的迅速傳播導致2019年嚴重特殊傳染性肺炎(COVID-19)大流行,根據世界衛生組織報告:到2021年10月,超過二億三千五百萬人被感染,481萬人死亡。隨著冬天的到來,世界正面臨另一波新冠變異病毒株感染的衝擊。
中央研究院細胞與個體生物研究所特聘研究員兼任生醫轉譯研究中心主任吳漢忠的研究團隊發表了一項研究成果,研究團隊研發出多株有潛力COVID-19治療性抗體(針對宿主受體結合區域-RBD之中和性抗體)對於新冠肺炎變異株的預防及治療都深具發展潛力。近期刊登在國際期刊《PLOS病原體》 (PLoS Pathogens)。
為了應對嚴峻形勢,全球已加速在研發新的療法來預防及治療,其中一個治療策略就是研發中和性抗體進行預防及治療,可以減少病毒載量以阻止疾病進展並加速患者康復。在廖俊智院長的整合及建立國內學研單位COVID-19合作平台下,團隊運用已建置治療性抗體平台,成功的開發COVID-19治療性抗體。
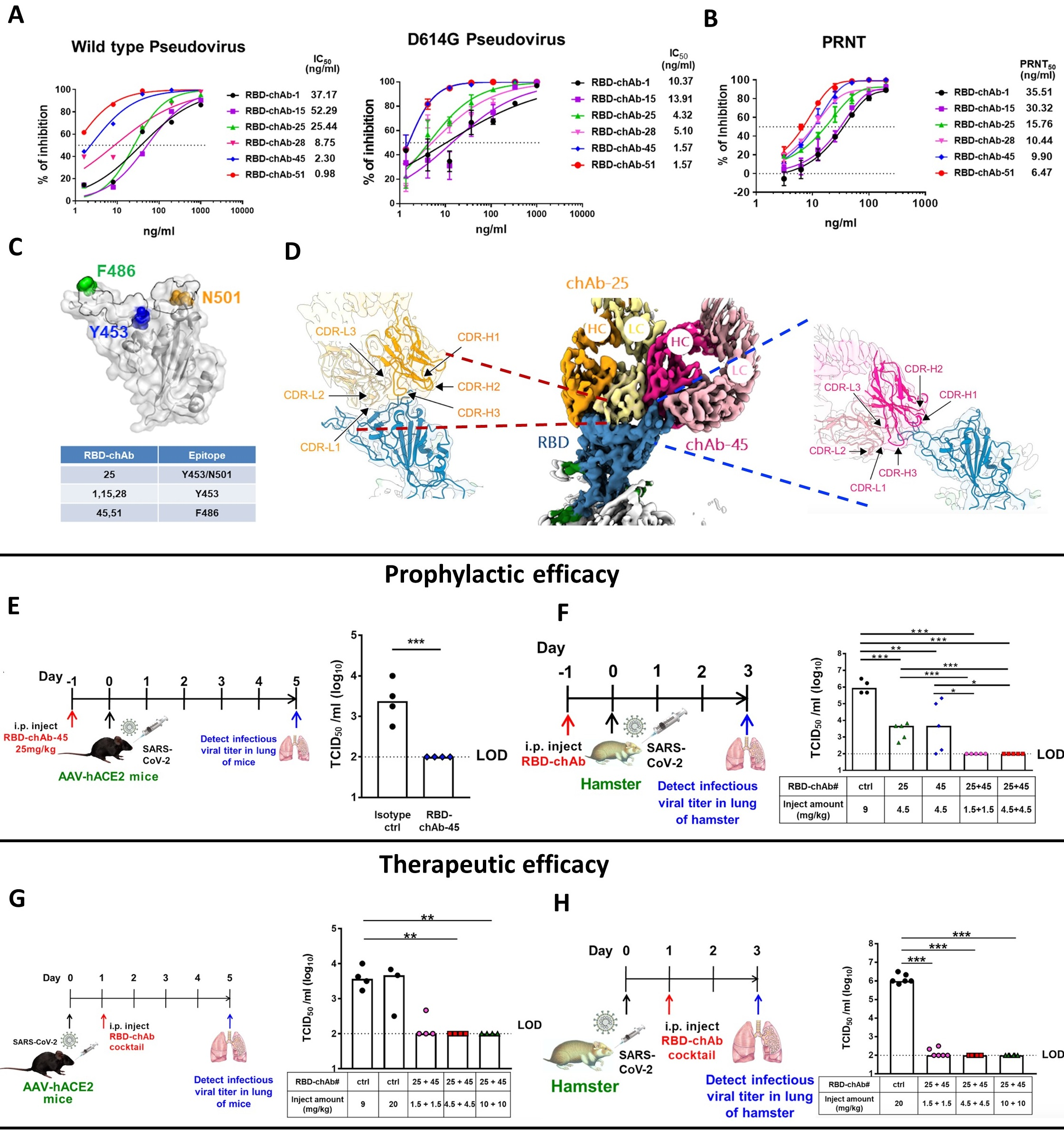
研究團隊利用小鼠融合瘤技術產生多株的抗SARS-CoV-2單株抗體。接著運用抗體工程-改造成人類嵌合型抗體(chAb)。進一步透過偽病毒中和測定(Pseudovirus assay)和SARS-CoV-2病毒進行了溶斑減少中和試驗(PRNT)驗證後。發現RBD-chAb-1,-15,-25,-28,-45和-51,這六個chAbs均顯示出對SARS-CoV-2有高度的中和作用。
吳漢忠表示:由於新冠肺炎屬於RNA病毒,容易伴隨突變而產生許多不同的變異病毒株,而這些變異病毒株往往導致疫苗和中和性抗體的保護力下降。於是利用新冠肺炎變異株的偽病毒來測試我們所發現六株具發展潛力中和性抗體的中和能力,其中的五株中和性抗體對於目前值得關注的變異株如:英國株、南非株、巴西株、加州株、紐約株、印度株等常見的變異病毒株的偽病毒皆有良好的中和力。
接著,蘇士傑和楊子靖進一步發現及釐清雙抗體聯合使用有更好的中和力對抗新冠肺炎變異病毒株。徐尚德表示:藉由結構生物學-冷凍電顯技術觀察到雙抗體RBD-chAb-15/45或RBD-chAb-25/45可同時結合到SARS-CoV-2的宿主受體結合區域-RBD。也發現雙抗體都有其個別在RBD的特定結合區域,所以不會造成抗體同時結合至RBD所形成的立體障礙。由此複合體結構狀態推測,能有效中和對抗新冠變異病毒株的逃逸,達到抗體雞尾酒療法。
研究團隊進一步使用抗體雞尾酒療法用來減少變異病毒株所產生的抗藥性,由我們六株中和性抗體所組合的雞尾酒療法對於常見的變異病毒株的偽病毒都顯示有良好中和效果。在感染新冠病毒的動物實驗中:也發現中和性抗體所組合的雞尾酒療法RBD-chAb-15/45或RBD-chAb-25/45雙抗體可有效預防及治療小鼠及倉鼠對於SARS-CoV-2之感染。
吳漢忠表示:「這六種嵌合抗體可以策略式地研發多種的抗體雞尾酒療法。可以預見這項研究成果,未來將被廣泛應用在SARS-CoV-2 突變株的各種預防及治療。」目前,此六種COVID-19治療性抗體對於新型冠狀病毒的預防及保護都深具發展潛力,已申請國內外專利,未來可望化研為用,造福人群。
此論文共同第一作者為中研院細生所蘇士傑博士及生化所楊子靖博士生。研究團隊包含中研院細生所及轉譯中心吳漢忠、生化所徐尚德、生醫所及轉譯中心林宜玲、生醫所及轉譯中心陶秘華及基因體研究中心詹家琮。此外,研究團隊因研發出有潛力COVID-19治療性抗體,已經發表三篇文章在國際期刊,兩篇刊登在《PLOS病原體》以及一篇刊登在《自然-結構與分子生物學》。
經費來源包括科技部和中研院。
Therapeutic Antibody Cocktail for Prevention and Treatment of COVID-19
During the COVID-19 pandemic, more than 235 million people have been infected with SARS-CoV-2 and 4.81 million are dead as of June 2021 (WHO, 2021). With the recent emergence of more infectious viral variants, the world may soon face a resurgence of the disease. While vaccine availability is becoming more and more widespread, new drugs are still necessary to prevent and treat infections.
In a recent collaborative study at Academia Sinica, researchers generated a series of chimeric antibodies that can neutralize SARS-CoV-2 by binding to the spike protein receptor-binding domain (RBD). Two of the identified antibodies were combined in a cocktail therapy that shows remarkable ability to attenuate viral infection and disease progression in animal models. The study was conducted by the COVID-19 Research Group at Academia Sinica, which is spearheaded by Prof. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology and Biomedical Translation Research Center (BioTReC). The work will be published in the world-renowned journal, PLoS Pathogens.
Over the past 18 months, the global research community has made extraordinary efforts to develop novel treatments for COVID-19. To facilitate these efforts in Taiwan, the president of Academia Sinica, James C. Liao, assembled a cooperative platform for academic research on COVID-19. The COVID-19 Research Group utilized this platform for therapeutic antibody development, taking advantage of facilities for antibody screening, target identification, and antibody engineering to optimize the molecules. The study authors credited this streamlined organization as being key to their successful development of therapeutic COVID-19 antibodies.
In their study, the COVID-19 Research Group first used mouse hybridoma technology to develop a number of potential antibodies against the SARS-CoV-2 spike protein RBD. The group then successfully humanized several of the mouse monoclonal antibodies, including RBD-chAb-1, -15, -25, -28, -45 and -51, while ensuring that the binding activities and biological functions were retained. The experiments were mostly performed by the two lead co-authors, Dr. Shih-Chieh Su and Mr. Tzu-Jing Yang.
The senior author, Prof. Wu, emphasized the importance of using the antibodies in combination, saying “SARS-CoV-2 is an RNA virus with a high mutation rate that quickly leads to the development of variants. Some of those variants may be resistant vaccines and neutralizing antibodies. In this study, we used SARS-CoV-2 variant pseudoviruses to verify that the neutralizing abilities of our RBD-chAbs remained high for the most common mutation variants, including the United Kingdom variant, South African variant, Brazil variant, California variant, New York variant and India variant. Since antibody cocktails are often useful to prevent the spread of drug-resistant SARS-CoV-2 variants, we ensured that our cocktail exhibits high neutralizing activities against all tested SARS-CoV-2 variant pseudoviruses.”
The head of a collaborating laboratory, Prof. Shang-Te D. Hsu, further clarified the reason that the two antibodies work well in combination. Prof. Hsu explained, “According to our Cryo-EM analysis, both chAbs in the cocktail, RBD-chAb-15/45 and RBD-chAb-25/45, can simultaneously bind to the RBD of SARS-CoV-2. This can occur because the two therapeutic chAbs target distinct epitopes within the RBD of SARS-CoV-2 spike protein. The simultaneous binding should increase the therapeutic efficacy and decrease the potential for virus escape mutants. It is also important to note that we confirmed the efficacy of our antibody cocktail as both a prophylactic agent and a post-infection treatment in animal models.”
According to Prof. Wu, all six chimeric antibodies can be used to strategically create cocktail therapies against multiple SARS-CoV-2 mutant strains. With the emergence of more contagious variants of SARS-CoV-2, such antibody cocktails hold great promise as tools to control disease and prevent the further emergence of drug-resistant variants.
Dr. Shih-Chieh Su from the Institute of Cellular and Organismic Biology, Academia Sinica, and PhD candidate Tzu-Jing Yang from the Institute of Biological Chemistry, Academia Sinica, are the co-first authors of this paper. The research teams include Dr. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology and Biomedical Translation Research Center (BioTReC), Dr. Shang-Te D. Hsu’s group at the Institute of Biological Chemistry, Drs. Mi-Hua Tao and Yi-Ling Lin’s group at the Institute of Biomedical Sciences and BioTReC, and Dr. Jia-Tsrong Jan’s group at the Genomics Research Center, Academia Sinica. The research teams have published three articles on this work, two in PLoS Pathogens and one in Nature Structural & Molecular Biology. The research teams thank Academia Sinica and the Ministry of Science and Technology for their support and research funding.
Article: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009704
Figure legend. The successful development of therapeutic COVID-19 antibody. The six-humanized monoclonal antibodies were generated, including RBD-chAb-1, -15, -25, -28, -45 and -51. Each had high binding activity and biological function. The antibodies in the cocktail RBD-chAb-25/45 can simultaneously bind to the receptor binding region (RBD) of SARS-CoV-2, according to the Cryo-EM analysis (upper). The efficacy of this antibody cocktail was tested in SARS-CoV-2-infected mouse and hamster models as prophylactic and post-infection treatments (middle and bottom).