Lab of Marine Molecular Biology and Biotechnology
-
 Jen-Leih WuDistinguished Visiting Fellow
Jen-Leih WuDistinguished Visiting Fellow- SpecialtyMolecular Biology, Molecular Virology, Developmental Biolog
- E-mailjlwu@gate.sinica.edu.tw
- Tel02-2789-9568
- LabR302/ICOB

Research achievements
The transgenic zebrafish as a model to study liver organogenesis and disease mechanism:
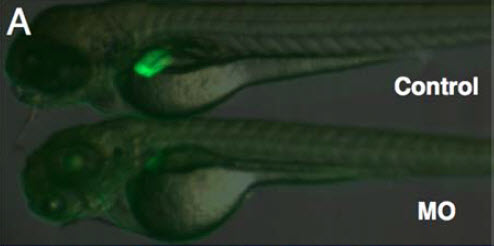
Progranulin (PGRN) is postulated to play a critical role in regulating pathological liver growth. Nevertheless, the exact regulatory mechanism of PGRN in relation to its functional role in embryonic liver development remains to be elucidated. In our study, the knockdown of Progranulin A (GrnA), an orthologue of mammalian PGRN resulted in impaired liver morphogenesis in zebrafish (Danio rerio). The vital role of GrnA in hepatic outgrowth and not in liver bud formation was further confirmed using whole- mount in situ hybridization markers. In addition, a GrnA deficiency was also found to be associated with the deregulation of MET-related genes in the neonatal liver using a microarray analysis. Therefore, our data have indicated that GrnA plays a vital role in embryonic liver morphogenesis in zebrafish. As a result, a novel link between PGRN and MET signaling is proposed (Li et al., 2010).
-
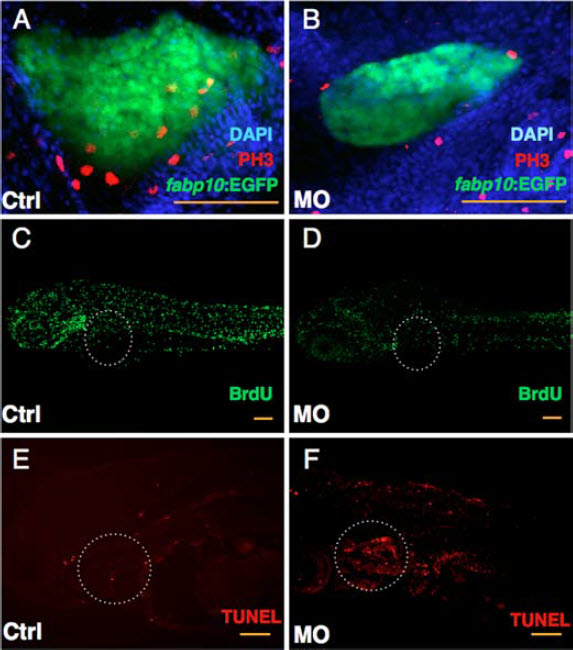
Figure 2. Knockdown of GrnA impairs liver cell proliferation and enhances apoptosis -
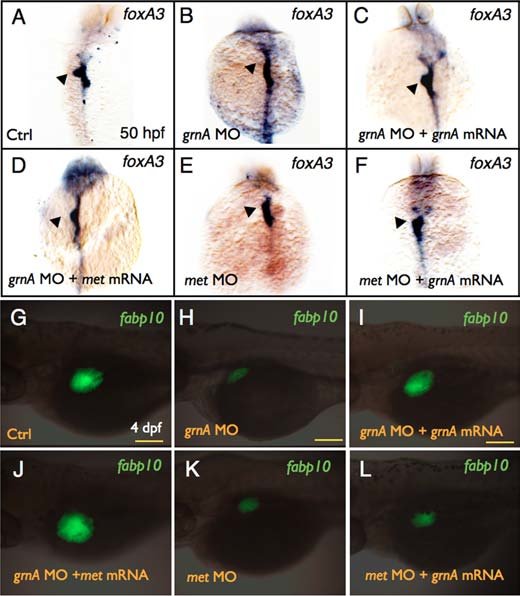
Figure 3. Effect of grnA and met on liver formation Whole-mount in situ hybridization of foxA3 expression in 50-hpf embryos injected with control MO (A), grnA MO (B), grnA MO with grnA (C) or met (D) mRNA, met MO (E), and met MO with grnA mRNA (F). EGFP expression in 4-dpf Tg(fabp10:EGFP) embryos that were injected with control MO (G), grnA MO (H), grnA MO with grnA (I) or met (J) mRNA, met MO (K), and met MO with grnA mRNA (L) (dorsal views, anterior up). The liver is indicated by the arrowhead. Ctrl, control. Scale bars, 100 μm.
we conditionally co-expressed hepatitis B virus X (HBx) and hepatitis C virus core (HCP) proteins in zebrafish livers, which caused fibrosis and consequently contributed to ICC formation at the age of 3 months. Suppressing the transgene expression by doxycycline (Dox) treatment resulted in the loss of ICC formation. The biomarker networks of zebrafish ICC identified by transcriptome sequencing and analysis were also frequently involved in the development of human neoplasms. The profiles of potential biomarker genes of zebrafish ICC were similar to those of human cholangiocarcinoma. Our data also showed that the pSmad3L oncogenic pathway was activated in HBx and HCP-induced ICC and included phosphorylation of p38 mitogen-activated proteinbase (MAPK) and p44/42 mitogen-activated protein kinase (ERK1/2), indicating the association with transforming growth factor beta 1 (TGF-b1) signaling pathway in ICC. Bile duct proliferation, fibrosis, and ICC were markedly reduced by knockdown of TGF-b1 by in vivo morpholinos injections. These results reveal that TGF-b1 plays an important role in HBx- and HCP-induced ICC development (Liu et al., 2012). This in vivo model is a potential approach to study the molecular events of fibrosis and ICC occurring in HBV and HCV infection.
- Figure 4. Liver-specific inducible transgenic zebrafish. A, Fluorescence photomicrograph of 3-month-old HBx+HCP zebrafish. B, Whole mount in situ hybridization of transgenic lines with HBx and HCP antisense probes (HBx-AS and HCP-AS, respectively).
- Figure 5. Histological examination of ICC induced by the co-expression of HBx and HCP. A, Vacuolated cytoplasm (left panel), bile duct cell hyperplasia (middle panel), and fibrosis (right panel) were detected in the liver of one-month-old HBx+HCP zebrafish. ICC was detected in the zebrafish liver at 3 months of age (B). The arrow indicates the bile duct.
- Figure6. Regulatory pathways in ICC tumorigenesis. From transcriptomic and histologic analysis demonstrated that activation of the TGF-β/pSmad3L oncogenic pathway and PGRN/MET signaling in 3 months HBx+HCP fish liver.
| Name | Job Title | Telephone | Remark | |
|---|---|---|---|---|
| Jen-Leih Wu | Distinguished Research Fellow | 02-2789-9534 | jlwu@gate.sinica.edu.tw | |
| Wangta Liu | Postdoctoral Fellow | 02-2789-9534 | liuwangta@yahoo.com.tw | |
| Sung-Yu Wu | Postdoctoral Fellow | 02-2789-9534 | joeywu1112@gmail.com | |
| Chih-Lu Wu | Postdoctoral Fellow | 02-2789-9573 | jwu8@ms14.hinet.net | |
| Gen-Hwa Lin | Research Assistant | 02-2789-9534 | genhwa@gate.sinica.edu.tw | |
| Tzu-Hsuan Yang | Project Manager | 02-2789-9534 | thyang@gate.sinica.edu.tw | |
| Yen-Chun Chen | Research Assistant | 02-2789-9534 | shiny_0224@yahoo.com.tw | |
| Hsu-Yu Chen | Research Assistant | 02-2789-9534 | dolphinazure@hotmail.com | |
| Ming-Ying Lee | Research Assistant | 02-2789-9534 | qwe80049@yahoo.com.tw | |
| Jing-Ruei Chi | Ph.D Candidate | 02-2789-9534 | jrc@firdi.org.tw | |
| Shin-Jie Huang | Ph.D Candidate | 02-2789-9534 | shinjiehuang1@gmail.com | |
| Ya-Wen Li | Ph.D Candidate | 02-2789-9534 | liyawen@gate.sinica.edu.tw | |
| Keng-Yu Chiang | Ph.D Student | 02-2789-9534 | windscent913@hotmail.com | |
| Chi-Hsueh Lin | Master Student | 02-2789-9534 | micheal30025005@gmail.com | |
| Yuang-Chung Sung | Master Student | 02-2789-9534 | dying.grace@gmail.com | |
| Ciao-Yi Chang | Master Student | 02-2789-9534 | r00b45012@ntu.edu.tw | |
| Chih- Lun Cheng | Master Student | 02-2789-9534 | skyclear1120@hotmail.com | |
| See- Ting Ng | Master Student | 02-2789-9534 | jenny-nst87@hotmail.com | |
| Hsu-Chia Huang | Master Student | 02-2789-9534 | joseph03111987@hotmail.com | |
| wei-lin Teng | Master Student | 02-2789-9534 | 10136003@ntou.edu.tw | |
| Chia-ray Lin | Postdoctoral Fellow | 02-2789-9534 | cjlin91@gmail.com |
-
Rekha, R.D., Amali, A.A., Her, G.M., Yeh, Y.H., Gong, H.Y., Hu, S.Y., Lin, G.H. and Wu, J.L.* (2008) Thioacetamide accelerate steatohepatitis, cirrhosis and HCC by expressing HCV core protein in transgenic zebrafish Danio rerio. Toxicology, 243 : 11 - 22.
-
Lu, M.W., Chao, Y.M., Guo, T.C., Santi, N., Evensen, Ø., Kasani, S.K., Hong, J.R. and Wu, J.L.* (2008) The interferon response is involved in nervous necrosis virus acute and persistent infection in zebrafish infection model. Molecular Immunology, 45 : 1146 - 1152.
-
Lin, C.J.F., Gong, H.Y., Tseng, H.C., Wang, W.L. and Wu, J.L.* (2008) miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochemica l and Biophysical Research Communications, 375 : 315 - 320.
-
Hu, S.Y., Lin, P.Y., Liao, C.H., Gong, H.Y., Lin, G.H., Kawakami, K. and Wu, J.L.* (2010) Nitroreductase-mediated gonadal dysgenesis for infertile control of genetically modified zebrafish. Marine Biotechnology, 12 : 569 - 578.
-
Li, Y.H., Chen, H.C., Hu, S.Y., Li, Y.W., Lin, G.H., Lin, C.C., Gong, H.Y., Liu, W. and Wu, J.L.* (2010) Progranulin A-mediated MET signaling is essential for liver morphogenesis in zebrafish. Journal of Biological Chemistry, 285 : 41001 - 41009.
-
Hu, S.Y., Liao, C.H., Lin, Y.P., Li, Y.H., Gong, H.Y., Lin, G.H., Kawakami, K., Yang, T.H. and Wu, J.L.* (2011) Zebrafish eggs used as bioreactors for the production of bioactive tilapia insulin-like growth factors.Transgenic Research, 20 : 73 - 83.
-
Wang, W.L., Hong, J.R., Lin, G.H., Liu, W., Gong, H.Y., Lu, M.W., Lin, C.C. and Wu, J.L.* (2011) Stage-Specific expression of TNFα regulates Bad/Bid mediated apoptosis and RIP1/ROS-mediated secondary necrosis in birnavirus-infected fish cells. PLoS ONE, 6 : e16740.
-
Wang, W.L., Liu, W., Gong, H.Y., Hong, J.R., Lin, C.C. and Wu, J.L.* (2011) Activation of cytokines expression occurs through the TNFα/NF-κB-mediated pathway inbirnavirus infected cells. Fish and Shellfish Immunology, 31 : 10 – 21.
-
Hu, S.Y., Tai, C.C., Li, Y.H. and Wu, J.L.* (2012) Progranulin compensates for blocked IGF-1 signaling to promote myotube 3 hypertrophy in C2C12 myoblasts via the PI3K/Akt/mTOR pathway. FEBS Letters, 586 : 3485 – 3492.
-
Liu, W., Chen, J.R., Hsu, C.H., Li, Y.S., Chen, Y.M., Lin, C.Y., Huang, S.J., Chang, Z.K., Chen, Y.C., Lin, C.H., Gong, H.Y., Lin, C.C., Kawakami, K. and Wu, J.L.* (2012) A zebrafish model of intrahepatic cholangiocarcinoma by dual expression of hepatitis B virus X and hepatitis C virus core protein in liver. Hepatology, 56 : 2268 – 2278.
-
Lin, M.C., Hui, C.F., Chen, J.Y.* and Wu, J.L.* (2012) The antimicrobial peptide, shrimp anti-lipopolysaccharide factor (SALF), inhibits proinflammatory cytokine expressions through the MAPK and NF-κB pathways in Trichomonas vaginalis adherent to HeLa cells. Peptide, 38 : 197-207.
-
Huang, C. W., Li, Y.H., Hu, S.Y., Chi, J.R., Liao, C.H., Lin, G.H., Lin, C.C., Gong, H.Y., Chen, R.H., Chang, S.J., Liu, F.G. and Wu, J.L.* (2012) Differential expression patterns of growth-related microRNAs in the skeletal muscle of Nile Tilapia (Oreochromis niloticus). Journal of Animal Science, 90 : 1-14.