Appendageal structures speed up wound healing response in adult zebrafish
- Date:
Highly regenerative vertebrates like teleost zebrafish and urodele amphibians are extremely efficient at healing large-sized wounds. Within only a few hours after major injury, these animals can re-establish skin barrier function to prevent opportunistic infections and excessive inflammation; this action also helps to initiate robust regenerative programs when complex tissues and organs are damaged. In sharp contrast, the wound healing/re-epithelialization process can take several weeks to complete in less regenerative animals (e.g., humans and mice). Thus, understanding how and why the rapid re-epithelialization process is possible in highly regenerative animals could provide insights that suggest new strategies for skin tissue engineering, repair and regeneration.
Here, we developed a macroscopic live imaging platform for capturing a total of ~60,000 Superficial Epithelial Cells (SECs) in one single field of view (44.22 mm2). Using this platform for whole-animal monitoring of the entire re-epithelialization process on distinct body regions, we first determined that wound-adjacent SECs migrate at different speeds. Unexpectedly, we then found that the Fin-resident Epithelial Cells (FECs) are highly mobile and migrate to cover proximal body wounds, regardless of the type of fin. Upon wounding, as many as 55% of the FECs readily mobilize on a whole-organ scale, with some portion of the mobilized cells invading neighboring body regions. Remarkably, these relocated FECs are not eliminated once the wound is closed; instead, the cells can survive for one year or more on the occupied body surface. By examining both ‘fin-less’ and ‘fin-gaining’ individuals, we determined that the FEC-supplying fins play a pivotal role in determining the speed of wound closure. We further identified that FECs reside on a basement membrane with a distinct extracellular matrix profile. In particular, the activity of the extracellular matrix component lamb1a governs FEC migration by enabling the formation of lamellipodia in these cells.
In summary, our macroscopic live imaging approach has enabled us to uncover a potential reservoir of reparative cells capable of self-replenishment and regeneration. These findings assign a previously unrecognized physiological function to an appendageal structure that exists in around 50% of all vertebrate species. In addition, our study highlights the importance of body-wide, inter-organ communication and coordination as an efficient yet region-specific strategy for mobilizing numerous cells to support urgent tissue repair and regeneration.
The first author, Fiorency Santoso, is a research assistant in Dr. Chen’s laboratory. Other team members include Marco De Leon, Wei-Chen Kao, Wei-Chen Chu, Hsiao-Yuh Roan, Gang-Hui Lee, Ming-Jer Tang, Ji-Yen Cheng, and Chen-Hui Chen. This study is supported by funding from the Institute of Cellular and Organismic Biology and grants from Academia Sinica to C.-H.C. (AS-CDA-109-L03 and AS-GCS-112-L01); and grants from National Science and Technology Council, Taiwan, to C.-H.C. (NSTC 110-2628-B-001-016, and NSTC 111-2628-B-001-026).
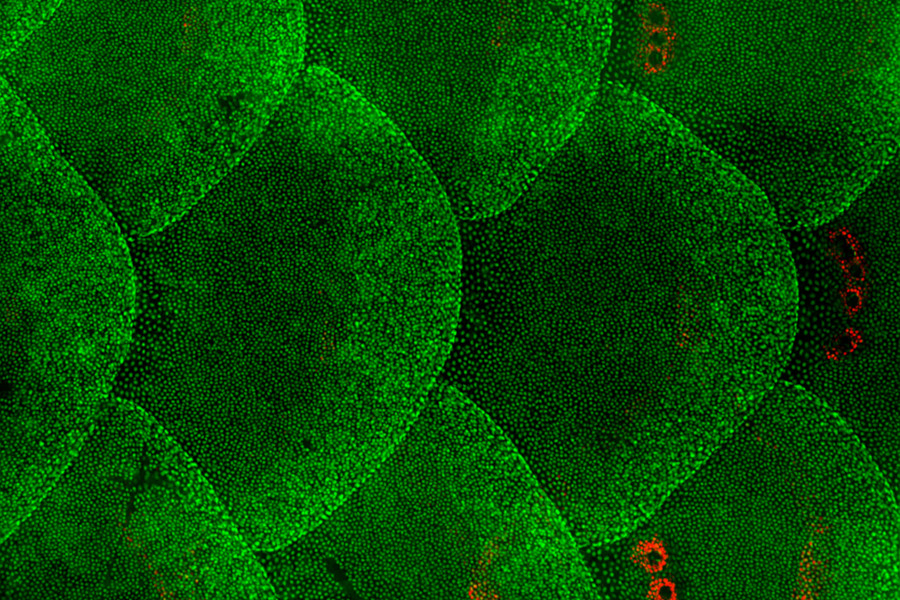
Figure 1 : The skin epithelial cells on the body surface of adult zebrafish.
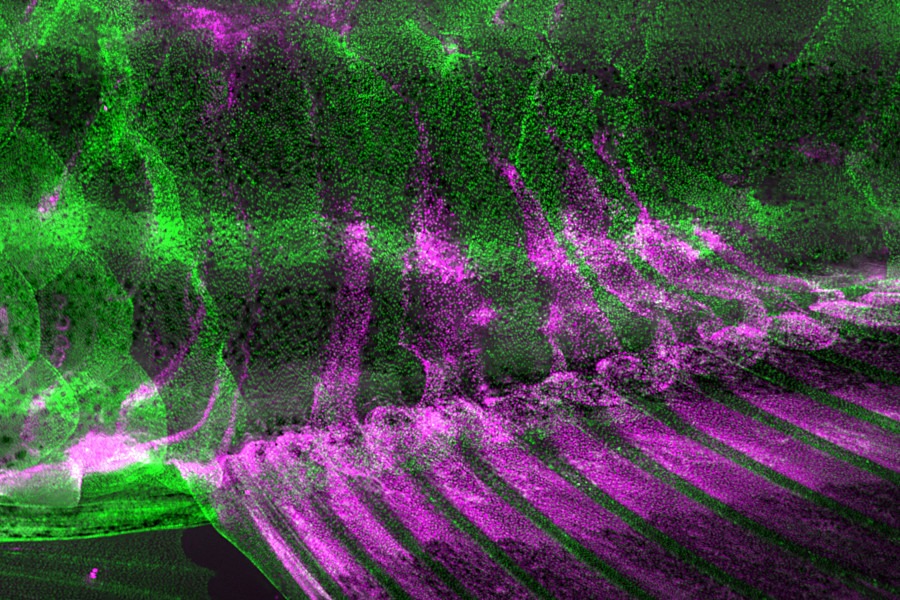
Figure 2 : The fin epithelial cells (shown in magenta) migrate to cover the body surface of adult zebrafish.
Article title: Appendage-resident epithelial cells expedite wound healing response in adult zebrafish
Article link: https://doi.org/10.1016/j.cub.2024.06.051