Congratulations for Dr. Han-Chung Wu granted 2022 Future Tech Award: COVID-19 Diagnosis and Therapy
- Date:
Congratulations for Dr. Han-Chung Wu granted 2022 Future Tech Award: COVID-19 Diagnosis and Therapy
The event “2022 Taiwan Innotech Expo – Future Tech Pavillon” has attracted almost 600 applications of the latest cutting-edge technologies important for industries developed by research teams from universities and research institutes throughout Taiwan. After fierce and stringent selection, 81 applications won the Future Tech Awards in 2022, with the world competitive technologies mainly in the themes of “Green Energy & Evolutionary Materials”, “Precise Health”, and “Artificial Intelligence of Things (AIoT) & Smart Applications”. Dr. Han-Chung Wu won the award in “Precise Health” with the technology solution developed for “COVID-19 Diagnosis and Therapy”. The technology developed not only attains scientific breakthrough, but also has good clinical and industrial applicability.
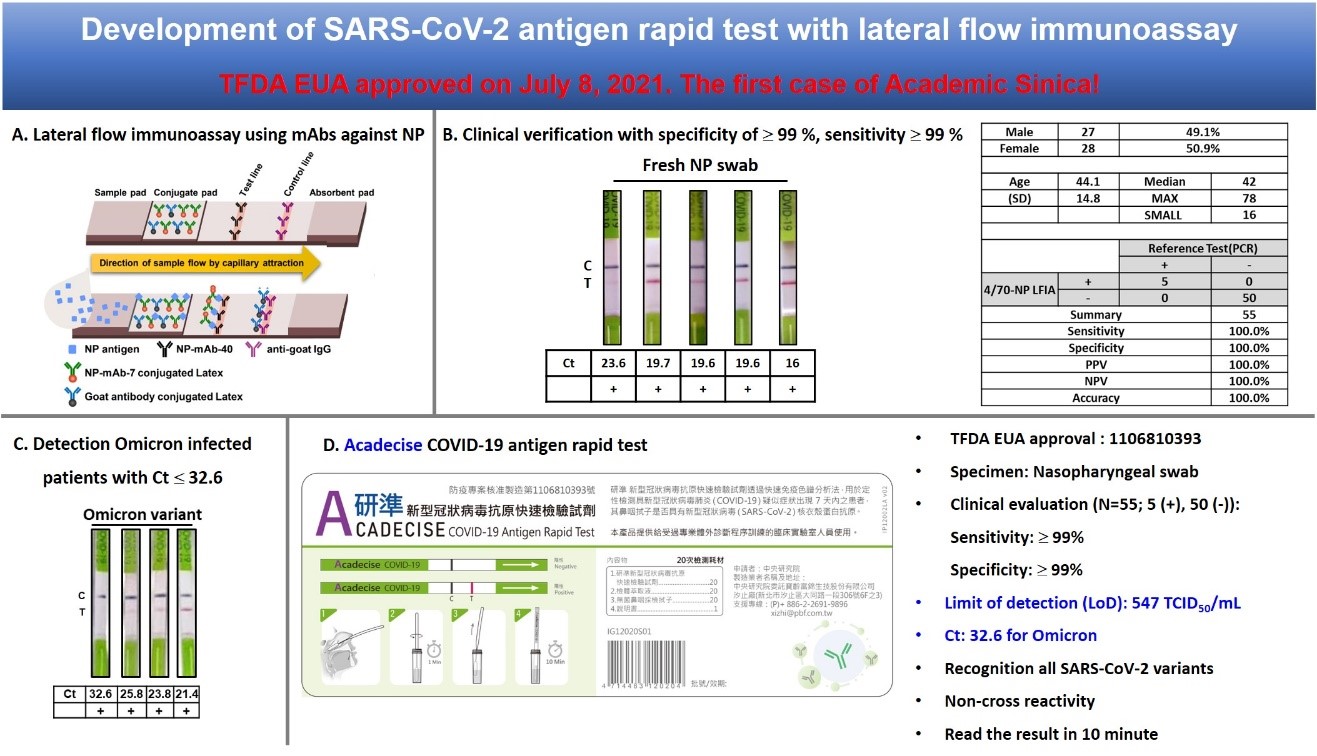
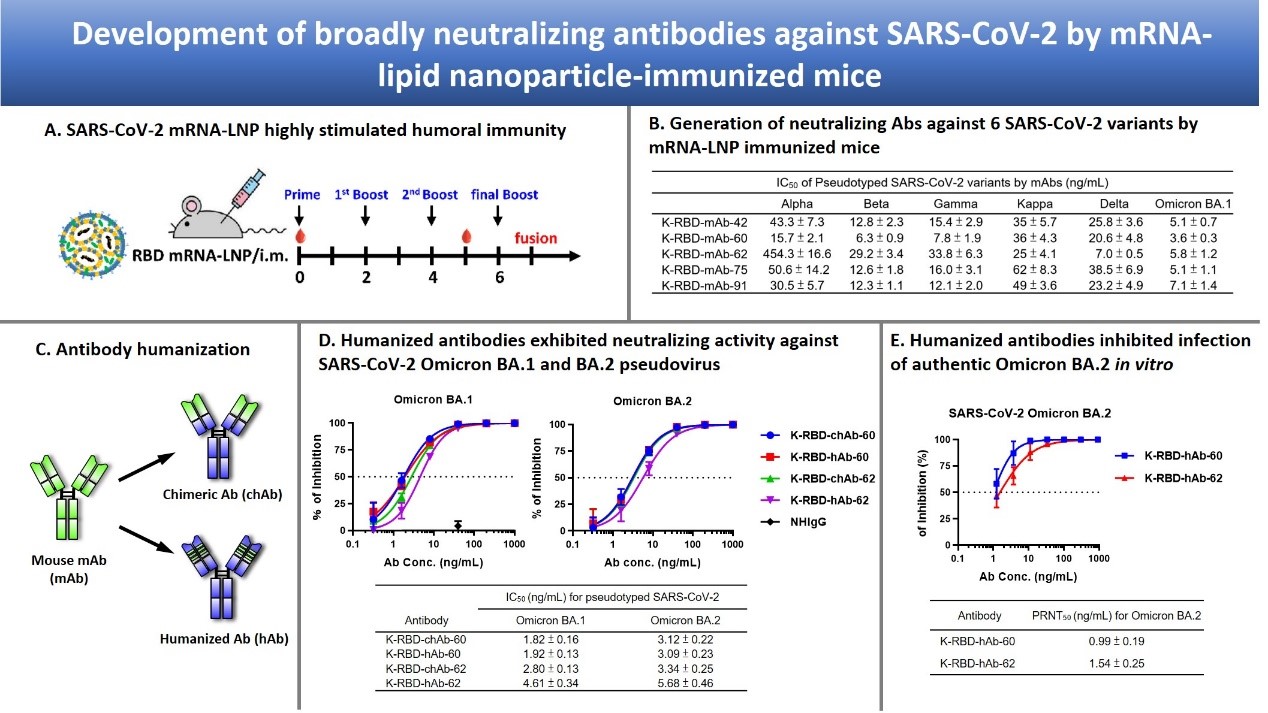
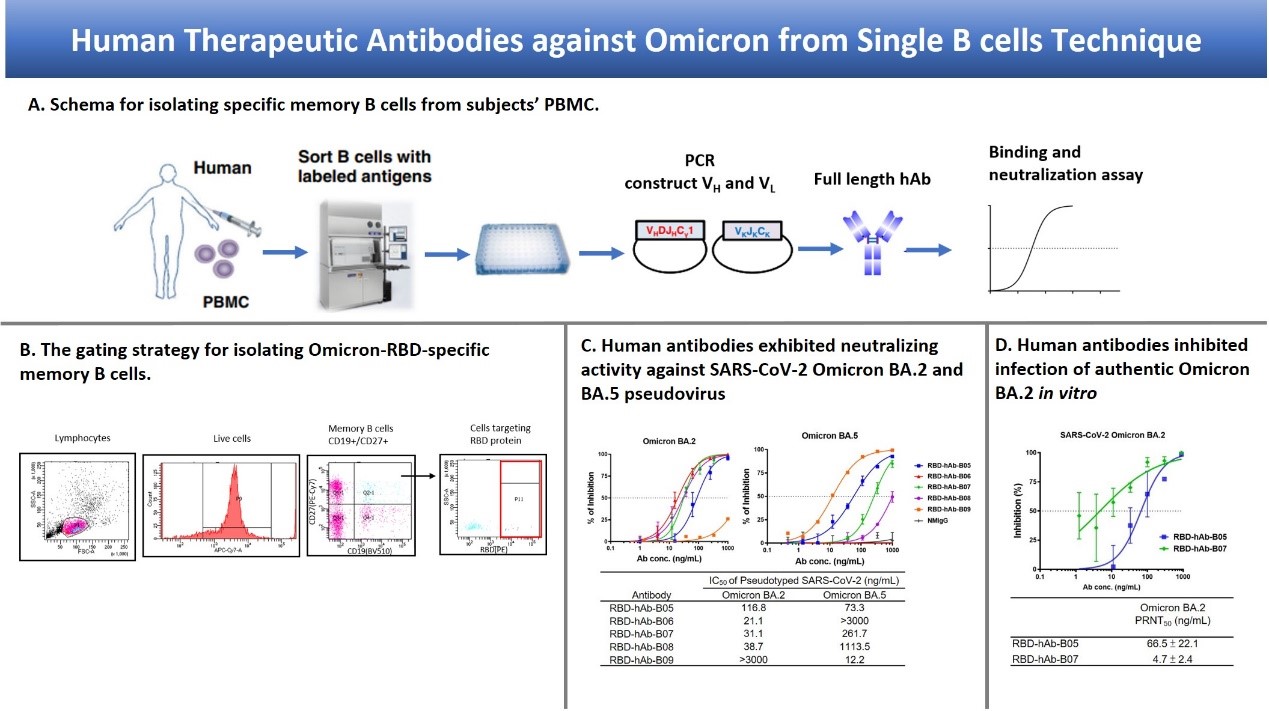
For detection of SARS-CoV-2, we generated mAbs against NP and used these mAbs to develop the antigen rapid test “Acadecise”. It showed high sensitivity in clinical trials and received the EUA from TFDA. To overcome the immune escape of SARS-CoV-2 Omicron variants, we used mRNA-lipid nanoparticle (LNP) immunization method to generate Omicron-targeting mAbs, two of which were engineered to humanized Abs for broadly neutralizing all variants of concern, including Omicron. We further utilized single B cell Ab platform to isolate human Abs (hAbs) against Omicron from vaccinated healthy donors. The hAbs showed the neutralizing potency to Omicron BA.2 and BA.4/5. The Abs we developed will be useful to detect or treat current SARS-CoV-2 variants.
Compared to the sensitivity of the US FDA-approved SARS-CoV-2 antigen rapid tests, Acadecise is listed 6th according to Limit of Detection. Thus, the sensitivity of Acadecise is internationally competitive. More importantly, clinical data showed that Acadecise enabled to detect Omicron-positive nasopharyngeal specimens with quite low virus loads (Ct ≤32.6). LNP-mediated delivery is a key technology of the successful implementation of mRNA-based vaccines. We used this time-saving approach to efficiently stimulate humoral immunity and rapidly generate broadly neutralizing Abs against all VOC. We also isolated Omicron-specific human Abs by a human single B Ab platform. The identified Abs exhibit low IC50 values against BA.1, BA.2 and BA.4/5.
The SARS-CoV-2 rapid antigen test “Acadecise” has been approved by the Taiwan Food and Drug Administration for emergency manufacturing. This is the first instance in which Academia Sinica has obtained a medical material license. After the manufacturing and distribution contract is signed, the test can be marketed for virus detection. In addition to being used for treatment, broad-spectrum neutralizing antibodies can also be used for prophylaxis, helping immunocompromised individuals to rapidly increase their concentrations of neutralizing antibodies and prevent the occurrence of mild to severe COVID-19.
Link: https://youtu.be/n45iHONvX20