ENO1 Promotes Lung Cancer Metastasis via HGFR and WNT Signaling–Driven Epithelial-to-Mesenchymal Transition
- Author:Hsin-Jung Li, Feng-Yi Ke, Chia-Ching Lin, Mei-Yi Lu, Yi-Huei Kuo, Yi-Ping Wang, Kang-Hao Liang, Shin
- Journal: Cancer Research Aug. 2021 Vol. 81, Issue 15 https://cancerres.aacrjournals.org/content/81/15/4094
Neutralizing ENO1 prevents lung cancer cell growth and metastasis
For many types of cancer, the cell-surface molecule ENO1 (α-enolase) is considered to be a promising target for new diagnostic tools and therapeutics. A recent study by Dr. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology, Academia Sinica, has greatly advanced our knowledge of how ENO1 promotes tumorigenesis and introduces a new tool for preventing cancer growth and metastasis. The study was published in Cancer Research [1].
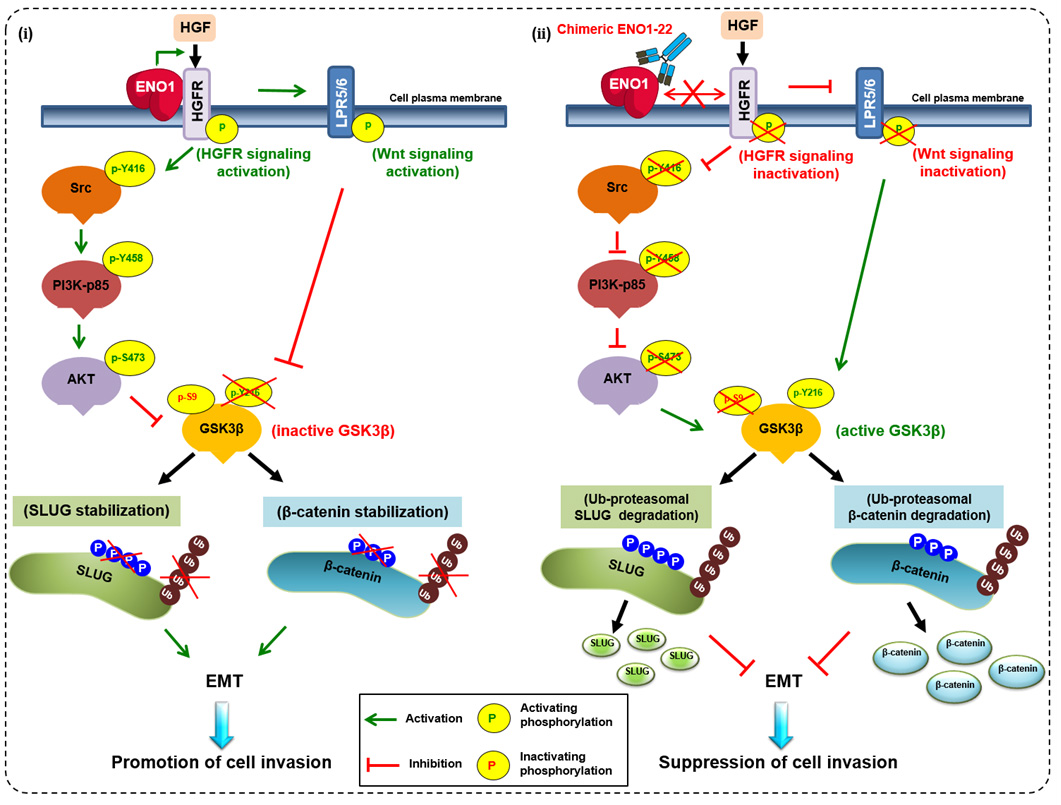
In their previous study, Wu’s group targeted ENO1 with a peptide called pHCT74. The authors incorporated pHCT74 into an innovative targeted drug delivery system that combines potent small molecule drugs with highly specific protein-targeting agents. This system leverages the benefits of the two therapeutic strategies and eliminates some of their key disadvantages, such as off-target toxicity. By increasing the therapeutic efficacies of small molecule drugs and reducing their side effects, the system can be used to improve treatments for many cancers in a previous study published in the top-ranking journal, Science Translational Medicine [2]. ENO1 is best known as a metabolic enzyme involved in the synthesis of pyruvate, but the protein is also highly expressed on the surface of cells in most tumors. This elevated expression of ENO1 in cancer cells is correlated with reduced survival rate and poor prognosis in many cancers. Wu’s group has studied the role of ENO1 in cancer and its potential as a therapeutic target for several years. Recently, Wu’s group showed that ENO1 activates two signaling pathways to stabilize the tumorigenic factor, SLUG, which then promotes epithelial-mesenchymal transition and invasion/metastasis in lung cancer. In this study, they further examined the biological role of ENO1 in tumor progression and invasion, demonstrating that ENO1-promoted lung cell invasion, tumor metastasis and progression specifically involves the HGFR-Src-PI3K-AKT-GSK3β-SLUG and HGFR-WNT-GSK3β-SLUG signaling axes. This mechanism of action suggests that in addition to its utility as a biomarker, ENO1 may also be a valuable target for lung cancer therapeutics.
In order to test the potential of ENO1 as a therapeutic target, the authors developed a neutralizing antibody, called chENO1-22, which is able to prevent ENO1 signaling. Application of chENO1-22 to cancer cells was found to decrease the level of SLUG, thereby reducing lung cancer cell growth and invasion. Importantly, this neutralizing antibody was also able to improve survival in a lung cancer xenograft mouse model, and it may be therefore be considered a promising therapeutic candidate for lung cancer. A US Provisional Application for Patent has been filed on the technology, as it is expected to be useful as a research tool and potentially as a clinically approved therapeutic in the future.
The current research paper, “ENO1 promotes lung cancer metastasis via HGFR and WNT signaling-driven epithelial-mesenchymal transition,” will be officially published on Aug 2nd, 2021. The full text of the paper: https://cancerres.aacrjournals.org/content/early/2021/06/17/0008-5472.CAN-20-3543. The first author of this paper is Dr. Hsin-Jung Li from the Institute of Cellular and Organismic Biology, Academia Sinica. The research team includes Distinguished Researcher Han-Chung Wu of the Institute of Cellular and Organismic Biology, Academia Sinica and Academician Pan-Chyr Yang of the Institute of Biomedical Sciences, Academia Sinica.
The studies by Dr. Han-Chung Wu and his group on the cellular mechanisms of ENO1-mediated tumorigenesis and the development of neutralizing antibodies were published in top journals. A working model depicts ENO1 promotion of cell invasion through regulation of the HGFR-Src-PI3K-AKT-GSK3β-SLUG and HGFR-WNT-GSK3β-SLUG axes. (ⅰ) ENO1 increases the phosphorylation of HGFR and Wnt co-receptor LPR5/6 and decreases GSK-3β activity to enhance SLUG stabilization and suppress E-cadherin expression. Ultimately, cancer cell invasion capacity is enhanced. (ⅱ) The chENO1-22 monoclonal antibody inhibits cancer cell invasion by blocking ENO1-HGFR axis-mediated downstream signaling to promote SLUG protein ubiquitination and degradation. Based on these discoveries and innovations, Dr. Wu’s group is poised to create new and effective treatments for cancer.
References:
- Li, H.J., et al., ENO1 promotes lung cancer metastasis via HGFR and WNT signaling-driven epithelial-mesenchymal transition. Cancer Res, 2021.
- Wu, C.H., et al., alpha-Enolase-binding peptide enhances drug delivery efficiency and therapeutic efficacy against colorectal cancer. Sci Transl Med, 2015. 7(290): p. 290ra91.