NOTCH1 Signaling Promotes Protein Stability of HER3 through the AKT Pathway in Squamous Cell Carcinoma of Head and Neck
- Author:Yi-Ping Wang, I-Ju Liu, Kai-Chi Chen & Han-Chung Wu
- Journal: Oncogenesis volume 10, Article number: 59 (2021), Springer Nature https://www.nature.com/articles/s41389-021-00348-5
NOTCH1 stabilizes HER3 in SCCHN
In a recent collaborative study by Dr. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology, Academia Sinica, and Dr. Yi-Ping Wang of the Department of Dentistry, National Taiwan University, the clinical importance of human epidermal growth factor receptor 3 (HER3) and its ligand, neuregulin (NRG1), were demonstrated. The study further unraveled the molecular interplay between NOTCH1 and HER3 and introduced a potential new strategy for stratification of patients to predict responsiveness to anti-HER3 targeted therapy. The study was published in Springer Nature Oncogenesis [1].
In the treatment of squamous cell carcinoma of head and neck (SCCHN), Epidermal growth factor receptor (EGFR) remains the sole molecular target of drugs with meaningful clinical benefit, except for those that target the PD1/PD-L1 pathway [2]. However, resistance to EGFR-targeted treatments in SCCHN can occur due to compensatory HER3 signaling and subsequent phosphatidylinositol-3-kinase (PI3K)/AKT activation [3]. Therefore, it is essential to understand the distribution and regulatory mechanisms of HER3 in SCCHN.
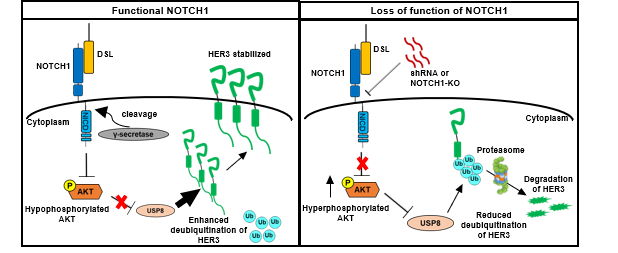
In this study, the authors explore the prevalence of HER3 expression and its distribution within SCCHN using immunohistochemical staining and clinicopathological correlation analyses. Concomitant high expression of HER3 and NRG1 in SCCHN was found to be associated with increased presence of regional lymphatic metastasis. The authors also noted that the majority of HER3-positive cells are among the differentiated cells in the center of the infiltrating tumor nests, while the NRG1-positive cells are primarily basaloid cells at the periphery of the cell nests. Starting with the knowledge that NOTCH1 is a master regulator in the terminal differentiation of the squamous epithelium [4] and that HER3 was mostly observed in more differentiated tumor cells, the authors then dissected the regulatory interplay between HER3 and NOTCH1. The investigation revealed that HER3 is positively regulated by NOTCH1 via transcriptional activation and inhibition of protein degradation. In particular, the degradation of HER3 is inhibited through the polyubiquitination machinery via AKT signaling and USP8 deubiquitinating enzyme. Further, loss of NOTCH1 function suppresses HER3 expression by increasing phosphorylation of AKT serine 473 in SCCHN cells, thereby promoting aggressiveness of the tumor cells.
The findings in this report indicate that the level of HER3 is regulated by NOTCH1 in SCCHN both transcriptionally and post-translationally, and NOTCH1 acts hierarchically upstream of the AKT pathway. Since NOTCH1 is inactivated in approximately 10% of SCCHN cases, this aberration would be expected to strongly impact the AKT pathway and diminish HER3. Therefore, exclusion of patients with NOTCH1-inactivated SCCHN may be beneficial for future clinical trials of HER3-targeting antibodies.
References:
- Wang, Y.P., et al., NOTCH1 signaling promotes protein stability of HER3 through the AKT pathway in squamous cell carcinoma of head and neck. Oncogenesis, 2021.
- Chow LQM., Head and neck cancer. N Engl J Med., 2021.
- Vlacich G, et al., Resistance to EGFR-targeted therapy: a family affair. Cancer Cell. 2011.
- Rothenberg SM, et al., The molecular pathogenesis of head and neck squamous cell carcinoma. J Clin Invest. 2012.