Structure-guided Antibody Cocktail for Prevention and Treatment of COVID-19
- Author:Shih-Chieh Su, Tzu-Jing Yang, Pei-Yu Yu, Kang-Hao Liang, Wan-Yu Chen, Chun-Wei Yang, Hsiu-Ting Lin,
- Journal: This paper was published in the PLOS Pathogens on October 21, 2021 https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009704
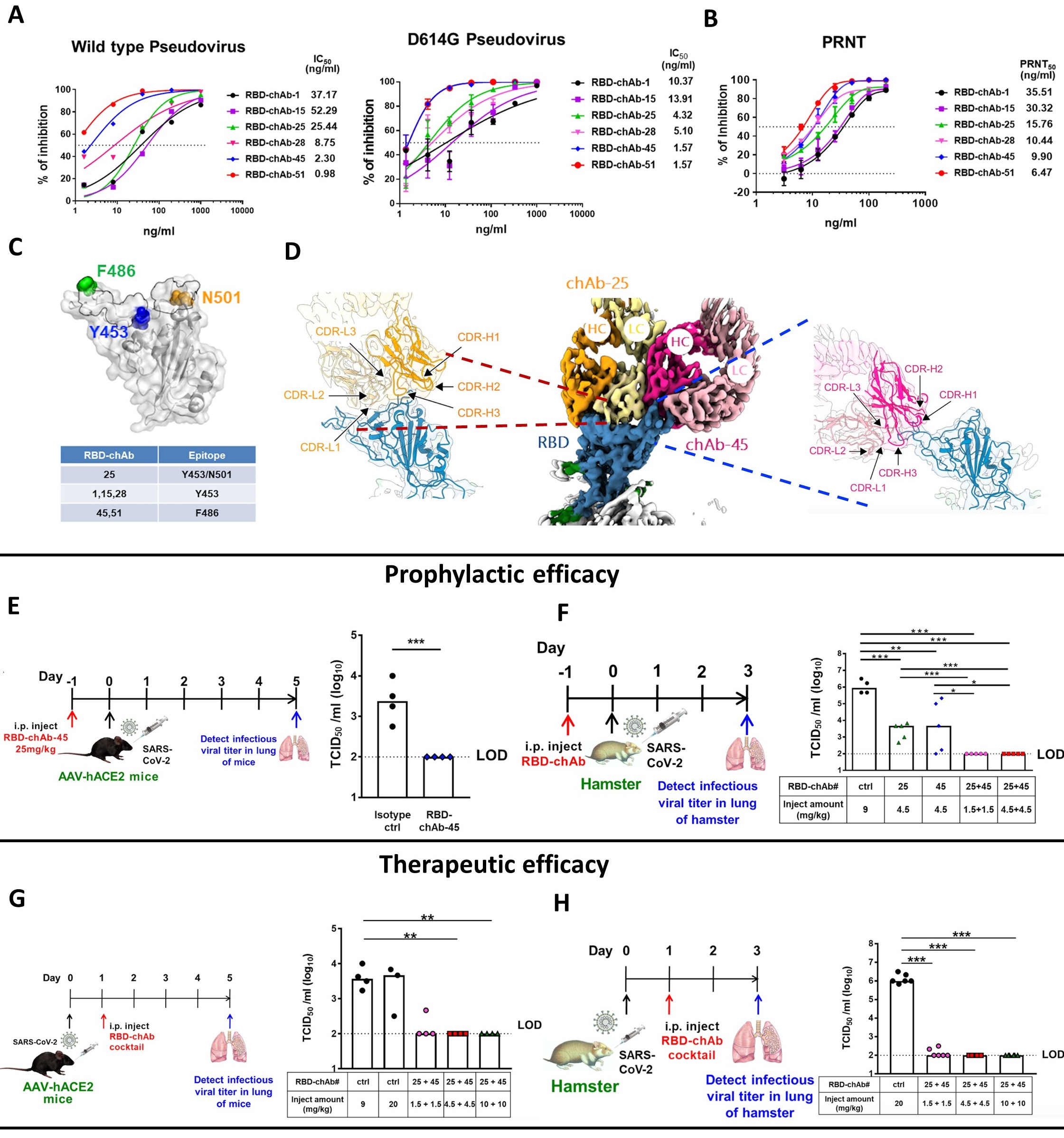
Figure legend. The successful development of therapeutic COVID-19 antibody. The six-humanized monoclonal antibodies were generated, including RBD-chAb-1, -15, -25, -28, -45 and -51. Each had high binding activity and biological function. The antibodies in the cocktail RBD-chAb-25/45 can simultaneously bind to the receptor binding region (RBD) of SARS-CoV-2, according to the Cryo-EM analysis (upper). The efficacy of this antibody cocktail was tested in SARS-CoV-2-infected mouse and hamster models as prophylactic and post-infection treatments (middle and bottom).
During the COVID-19 pandemic, more than 235 million people have been infected with SARS-CoV-2 and 4.81 million are dead as of June 2021 (WHO, 2021). With the recent emergence of more infectious viral variants, the world may soon face a resurgence of the disease. While vaccine availability is becoming more and more widespread, new drugs are still necessary to prevent and treat infections.
In a recent collaborative study at Academia Sinica, researchers generated a series of chimeric antibodies that can neutralize SARS-CoV-2 by binding to the spike protein receptor-binding domain (RBD). Two of the identified antibodies were combined in a cocktail therapy that shows remarkable ability to attenuate viral infection and disease progression in animal models. The study was conducted by the COVID-19 Research Group at Academia Sinica, which is spearheaded by Prof. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology and Biomedical Translation Research Center (BioTReC). The work will be published in the world-renowned journal, PLoS Pathogens.
Over the past 18 months, the global research community has made extraordinary efforts to develop novel treatments for COVID-19. To facilitate these efforts in Taiwan, the president of Academia Sinica, James C. Liao, assembled a cooperative platform for academic research on COVID-19. The COVID-19 Research Group utilized this platform for therapeutic antibody development, taking advantage of facilities for antibody screening, target identification, and antibody engineering to optimize the molecules. The study authors credited this streamlined organization as being key to their successful development of therapeutic COVID-19 antibodies.
In their study, the COVID-19 Research Group first used mouse hybridoma technology to develop a number of potential antibodies against the SARS-CoV-2 spike protein RBD. The group then successfully humanized several of the mouse monoclonal antibodies, including RBD-chAb-1, -15, -25, -28, -45 and -51, while ensuring that the binding activities and biological functions were retained. The experiments were mostly performed by the two lead co-authors, Dr. Shih-Chieh Su and Mr. Tzu-Jing Yang.
The senior author, Prof. Wu, emphasized the importance of using the antibodies in combination, saying “SARS-CoV-2 is an RNA virus with a high mutation rate that quickly leads to the development of variants. Some of those variants may be resistant vaccines and neutralizing antibodies. In this study, we used SARS-CoV-2 variant pseudoviruses to verify that the neutralizing abilities of our RBD-chAbs remained high for the most common mutation variants, including the United Kingdom variant, South African variant, Brazil variant, California variant, New York variant and India variant. Since antibody cocktails are often useful to prevent the spread of drug-resistant SARS-CoV-2 variants, we ensured that our cocktail exhibits high neutralizing activities against all tested SARS-CoV-2 variant pseudoviruses.”
The head of a collaborating laboratory, Prof. Shang-Te D. Hsu, further clarified the reason that the two antibodies work well in combination. Prof. Hsu explained, “According to our Cryo-EM analysis, both chAbs in the cocktail, RBD-chAb-15/45 and RBD-chAb-25/45, can simultaneously bind to the RBD of SARS-CoV-2. This can occur because the two therapeutic chAbs target distinct epitopes within the RBD of SARS-CoV-2 spike protein. The simultaneous binding should increase the therapeutic efficacy and decrease the potential for virus escape mutants. It is also important to note that we confirmed the efficacy of our antibody cocktail as both a prophylactic agent and a post-infection treatment in animal models.”
According to Prof. Wu, all six chimeric antibodies can be used to strategically create cocktail therapies against multiple SARS-CoV-2 mutant strains. With the emergence of more contagious variants of SARS-CoV-2, such antibody cocktails hold great promise as tools to control disease and prevent the further emergence of drug-resistant variants.
Dr. Shih-Chieh Su from the Institute of Cellular and Organismic Biology, Academia Sinica, and PhD candidate Tzu-Jing Yang from the Institute of Biological Chemistry, Academia Sinica, are the co-first authors of this paper. The research teams include Dr. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology and Biomedical Translation Research Center (BioTReC), Dr. Shang-Te D. Hsu’s group at the Institute of Biological Chemistry, Drs. Mi-Hua Tao and Yi-Ling Lin’s group at the Institute of Biomedical Sciences and BioTReC, and Dr. Jia-Tsrong Jan’s group at the Genomics Research Center, Academia Sinica. The research teams have published three articles on this work, two in PLoS Pathogens and one in Nature Structural & Molecular Biology. The research teams thank Academia Sinica and the Ministry of Science and Technology for their support and research funding.
Article: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009704