NOTCH1 Signaling Promotes Protein Stability of HER3 through the AKT Pathway in Squamous Cell Carcinoma of Head and Neck
- 作者:Yi-Ping Wang, I-Ju Liu, Kai-Chi Chen & Han-Chung Wu
- 期刊: Oncogenesis volume 10, Article number: 59 (2021), Springer Nature https://www.nature.com/articles/s41389-021-00348-5
NOTCH1 stabilizes HER3 in SCCHN
In a recent collaborative study by Dr. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology, Academia Sinica, and Dr. Yi-Ping Wang of the Department of Dentistry, National Taiwan University, the clinical importance of human epidermal growth factor receptor 3 (HER3) and its ligand, neuregulin (NRG1), were demonstrated. The study further unraveled the molecular interplay between NOTCH1 and HER3 and introduced a potential new strategy for stratification of patients to predict responsiveness to anti-HER3 targeted therapy. The study was published in Springer Nature Oncogenesis [1].
In the treatment of squamous cell carcinoma of head and neck (SCCHN), Epidermal growth factor receptor (EGFR) remains the sole molecular target of drugs with meaningful clinical benefit, except for those that target the PD1/PD-L1 pathway [2]. However, resistance to EGFR-targeted treatments in SCCHN can occur due to compensatory HER3 signaling and subsequent phosphatidylinositol-3-kinase (PI3K)/AKT activation [3]. Therefore, it is essential to understand the distribution and regulatory mechanisms of HER3 in SCCHN.
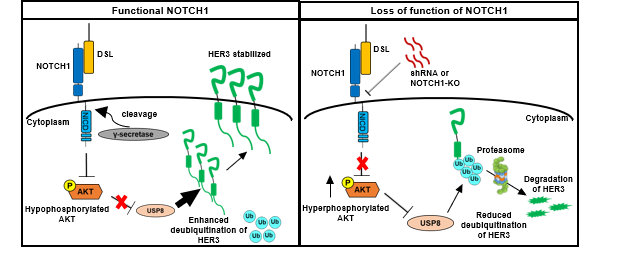
In this study, the authors explore the prevalence of HER3 expression and its distribution within SCCHN using immunohistochemical staining and clinicopathological correlation analyses. Concomitant high expression of HER3 and NRG1 in SCCHN was found to be associated with increased presence of regional lymphatic metastasis. The authors also noted that the majority of HER3-positive cells are among the differentiated cells in the center of the infiltrating tumor nests, while the NRG1-positive cells are primarily basaloid cells at the periphery of the cell nests. Starting with the knowledge that NOTCH1 is a master regulator in the terminal differentiation of the squamous epithelium [4] and that HER3 was mostly observed in more differentiated tumor cells, the authors then dissected the regulatory interplay between HER3 and NOTCH1. The investigation revealed that HER3 is positively regulated by NOTCH1 via transcriptional activation and inhibition of protein degradation. In particular, the degradation of HER3 is inhibited through the polyubiquitination machinery via AKT signaling and USP8 deubiquitinating enzyme. Further, loss of NOTCH1 function suppresses HER3 expression by increasing phosphorylation of AKT serine 473 in SCCHN cells, thereby promoting aggressiveness of the tumor cells.
The findings in this report indicate that the level of HER3 is regulated by NOTCH1 in SCCHN both transcriptionally and post-translationally, and NOTCH1 acts hierarchically upstream of the AKT pathway. Since NOTCH1 is inactivated in approximately 10% of SCCHN cases, this aberration would be expected to strongly impact the AKT pathway and diminish HER3. Therefore, exclusion of patients with NOTCH1-inactivated SCCHN may be beneficial for future clinical trials of HER3-targeting antibodies.
近期,中央研究院細胞與個體生物學研究所特聘研究員兼任生醫轉譯研究中心主任吳漢忠及台灣大學牙醫學院教授王逸平共同發表了一項研究,顯示於頭頸部鱗狀細胞癌(SCCHN)中,人類表皮生長因子受體3 (HER3)與其配體神經調節蛋白第一型(NRG1)同時高度表現時,與區域性淋巴轉移有關。同時團隊亦證實 NOTCH1路徑可穩定人類表皮生長因子受體3 (HER3)的蛋白表達,然當NOTCH1失活時,卻更進一步誘發頭頸部鱗狀細胞癌(SCCHN)細胞中AKT路徑活性,並促進腫瘤細胞的侵襲性。研究成果已發表在Springer Nature國際期刊Oncogenesis [1]。
至今,在SCCHN的臨床治療中,EGFR是除了PD1/PD-L1外唯一具臨床效益的標靶治療分子 [2]。然而,在經過一段時間治療後,卻發現癌細胞為了生存而產生令人棘手的抗藥性。而越來越多新研究表明在SCCHN中,代償性的HER3及下游PI3K/AKT傳導訊號的活化是使得癌細胞對於EGFR標靶治療產生抗藥性的關鍵角色 [3]。因此,揭示SCCHN中HER3的分佈及調控機轉便是重要議題。
在這項研究中,研究團隊先是評估了SCCHN中HER3和其配體NRG1的蛋白表達,並分析其與臨床數據的關聯。發現這兩種蛋白在SCCHN中同時高度表達與區域性淋巴轉移有關。有趣的是,HER3主要表達於浸潤性腫瘤中已分化的癌細胞群。在複層鱗狀上皮細胞分化成角質細胞中,主要由p63和NOTCH1所調控。而當角質細胞逐漸失去p63的表達時,NOTCH1則成為細胞命運的主要調控者 [4]。研究團隊近一步發現NOTCH1會促進HER3的轉譯,並透過AKT途徑和去泛素化酶(USP8)抑制其蛋白質降解。此外,研究團隊透過體外細胞實驗及體內動物腫瘤模型發現,NOTCH1功能的喪失雖抑制了HER3的表達,但增加了SCCHN細胞中AKT絲氨酸 473(S473)的磷酸化,更促進了腫瘤細胞的生長及侵襲。這些數據表明,不論是HER3的轉譯或轉錄後修飾,皆受NOTCH1所調控;並且在PI3K/AKT途徑的調控系統中, NOTCH1屬於更高位階的調控者。
基於以上新發現,研究團隊認為未來在HER3標靶治療的臨床試研中,若要達到最大的研究效益,可以事先排除NOTCH1功能失調的受試者;而NRG1的表達亦可作為評估參與此類試研受試者具代表性的生物標誌物。
本篇研究論文: NOTCH1 signaling promotes protein stability of HER3 through the AKT pathway in squamous cell carcinoma of head and neck 於2021年08月31日發表。論文全文: https://www.nature.com/articles/s41389-021-00348-5。研究團隊包含中研院細胞與個體生物學研究所吳漢忠特聘研究員及台灣大學牙醫專業學院王逸平教授。
研究團隊發現具功能性的NOTCH1信號抑制了AKT S473的磷酸化,從而使去泛素化酶USP8失去活性。隨後,激活的 USP8 會抑制 HER3的聚泛素化,進而阻止了HER3蛋白的蛋白酶體Proteasome降解(左圖)。當NOTCH1功能喪失時,AKT的磷酸化隨之增加,導致USP8的活性被下調。一旦去泛素化過程被抑制,多泛素化的HER3蛋白就會被Proteasome降解(右圖)。
References:
- Wang, Y.P., et al., NOTCH1 signaling promotes protein stability of HER3 through the AKT pathway in squamous cell carcinoma of head and neck. Oncogenesis, 2021.
- Chow LQM., Head and neck cancer. N Engl J Med., 2021.
- Vlacich G, et al., Resistance to EGFR-targeted therapy: a family affair. Cancer Cell. 2011.
- Rothenberg SM, et al., The molecular pathogenesis of head and neck squamous cell carcinoma. J Clin Invest. 2012.